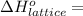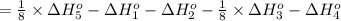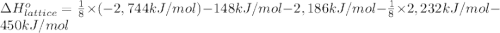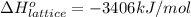Calculate the lattice energy of magnesium sulfide from the data given below. mg(s) → mg(g) δh° = 148 kj/mol mg(g) → mg2+(g) + 2e– δh° = 2186 kj/mol s8(s) → 8s(g) δh° = 2232 kj/mol s(g) + 2e– → s2–(g) δh° = 450 kj/mol 8mg(s) + s8(s) → 8mgs(s) δh° = –2744 kj/mol mgs(s)→mg2+(g) + s2–(g) δh°lattice = ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

You know the right answer?
Calculate the lattice energy of magnesium sulfide from the data given below. mg(s) → mg(g) δh° = 148...
Questions



Business, 10.12.2020 01:30







English, 10.12.2020 01:30


Engineering, 10.12.2020 01:30

Mathematics, 10.12.2020 01:30


Mathematics, 10.12.2020 01:30


History, 10.12.2020 01:30

English, 10.12.2020 01:30

Advanced Placement (AP), 10.12.2020 01:30

Medicine, 10.12.2020 01:30

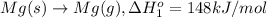 ..[1]
..[1]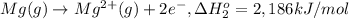 ..[2]
..[2] ..[3]
..[3]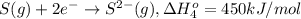 ..[4]
..[4]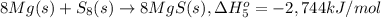 ..[5]
..[5]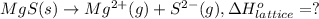 ..[6]
..[6]![[6]=\frac{1}{8}\times [5]-[1]-[2]-\frac{1}{8}\times [3]-[4]](/tpl/images/0407/1216/be747.png)