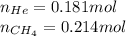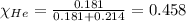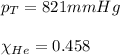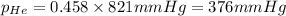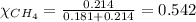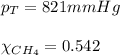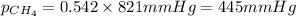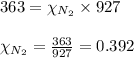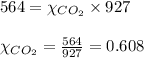Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of helium and 3.43 grams of methane. what is the partial pressure of each gas in the mixture?
phe = mm hg
pch4 = mm hg
2.) a mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm hg and carbon dioxide at a partial pressure of 564 mm hg. what is the mole fraction of each gas in the mixture?
xn2 =
xco2 =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

You know the right answer?
Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of heli...
Questions

Mathematics, 22.09.2021 20:50

Mathematics, 22.09.2021 20:50


Mathematics, 22.09.2021 20:50

English, 22.09.2021 20:50

Mathematics, 22.09.2021 20:50




Mathematics, 22.09.2021 20:50


Mathematics, 22.09.2021 20:50

English, 22.09.2021 20:50


History, 22.09.2021 20:50

Mathematics, 22.09.2021 20:50

Mathematics, 22.09.2021 20:50

Mathematics, 22.09.2021 20:50

Mathematics, 22.09.2021 20:50

English, 22.09.2021 20:50

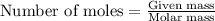 .....(1)
.....(1)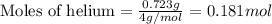
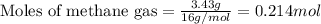
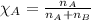 .......(2)
.......(2)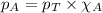 ......(3)
......(3)