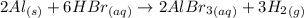Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
If 4.0 mol aluminum and 7.0 mol hydrogen bromide react according to the following equation, how many...
Questions


Social Studies, 27.09.2021 06:40

Physics, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40


Mathematics, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40






Mathematics, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40

Geography, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40

Advanced Placement (AP), 27.09.2021 06:40

Social Studies, 27.09.2021 06:40


Mathematics, 27.09.2021 06:40