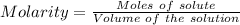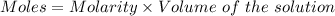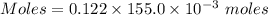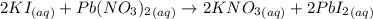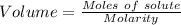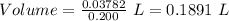Chemistry, 06.12.2019 20:31 milkshakegrande101
Potassium iodide reacts with lead(ii) nitrate in the following precipitation reaction:
2ki(aq)+pb(no3)2(aq)→2kno3(aq)+pbi2 (s)
what minimum volume of 0.200 m potassium iodide solution is required to completely precipitate all of the lead in 155.0 ml of a 0.122 m lead(ii) nitrate solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2


Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

You know the right answer?
Potassium iodide reacts with lead(ii) nitrate in the following precipitation reaction:
2ki(a...
2ki(a...
Questions




Mathematics, 20.08.2021 19:10

Mathematics, 20.08.2021 19:10


Chemistry, 20.08.2021 19:10


Biology, 20.08.2021 19:10

Mathematics, 20.08.2021 19:10


English, 20.08.2021 19:10


English, 20.08.2021 19:10




Computers and Technology, 20.08.2021 19:10


Mathematics, 20.08.2021 19:10