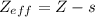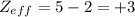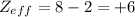Chemistry, 06.12.2019 20:31 jadepotts3965
The shielding of electrons gives rise to an effective nuclear charge,  , which explains why boron is larger than oxygen. estimate the approximate
, which explains why boron is larger than oxygen. estimate the approximate  felt by a valence electron of boron and oxygen, respectively? a. +5 and +8b. +3 and +6c. +5 and +6d. +3 and +8e. +1 and +4
felt by a valence electron of boron and oxygen, respectively? a. +5 and +8b. +3 and +6c. +5 and +6d. +3 and +8e. +1 and +4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
The shielding of electrons gives rise to an effective nuclear charge, [tex]z_{eff}[/tex], which expl...
Questions

Mathematics, 04.09.2020 20:01

Mathematics, 04.09.2020 20:01


Mathematics, 04.09.2020 20:01

Mathematics, 04.09.2020 20:01


Mathematics, 04.09.2020 20:01


Mathematics, 04.09.2020 20:01

Mathematics, 04.09.2020 20:01


English, 04.09.2020 20:01

English, 04.09.2020 20:01

History, 04.09.2020 20:01


Mathematics, 04.09.2020 20:01




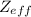 of the valence electron of boron and oxygen, we need to use the next equation:
of the valence electron of boron and oxygen, we need to use the next equation: