
Chemistry, 06.12.2019 19:31 anthonyvargas8780
Astandard nitric acid solution is prepared using 0.425 g of sodium carbonate, na2co3. the balanced equation is:
2 hno3 (aq) + na2co3 (s) ➝ 2 nano3 (aq) + h2o (l) + co2 (g)
referring to example exercise 2, provide in three significant figures:
a) # moles of na2co3
b) # moles of hno3
c) molarity of hno3 if 33.25 ml are required to reach a permanent endpoint.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

You know the right answer?
Astandard nitric acid solution is prepared using 0.425 g of sodium carbonate, na2co3. the balanced e...
Questions

Physics, 18.11.2019 11:31




English, 18.11.2019 11:31

Advanced Placement (AP), 18.11.2019 11:31

Health, 18.11.2019 11:31


Mathematics, 18.11.2019 11:31


Chemistry, 18.11.2019 11:31



Health, 18.11.2019 11:31


Mathematics, 18.11.2019 11:31

Chemistry, 18.11.2019 11:31

Mathematics, 18.11.2019 11:31

Biology, 18.11.2019 11:31

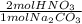 = 8.02x10⁻³ mol HNO₃
= 8.02x10⁻³ mol HNO₃

