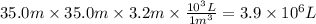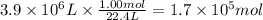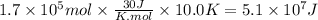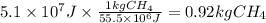Chemistry, 06.12.2019 17:31 keshewar2671
Assuming that all the energy given off in the reaction goes to heating up only the air in the house, determine the mass of methane required to heat the air in a house by 10.0 ∘c. assume each of the following: house dimensions are 35.0 m × 35.0 m × 3.2 m ; specific heat capacity of air is 30 j/k⋅mol; 1.00 mol of air occupies 22.4l for all temperatures concerned.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Assuming that all the energy given off in the reaction goes to heating up only the air in the house,...
Questions




Mathematics, 10.12.2021 14:00

Engineering, 10.12.2021 14:00


History, 10.12.2021 14:00

English, 10.12.2021 14:00





Mathematics, 10.12.2021 14:00

Mathematics, 10.12.2021 14:00

Physics, 10.12.2021 14:00

Mathematics, 10.12.2021 14:00

History, 10.12.2021 14:00

Advanced Placement (AP), 10.12.2021 14:00