

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Asample of hydrogen gas has an initial pressure of 2.86 atm and an initial volume of 8472 ml if the...
Questions


Spanish, 14.10.2019 18:30


Business, 14.10.2019 18:30

Mathematics, 14.10.2019 18:30

Mathematics, 14.10.2019 18:30

Social Studies, 14.10.2019 18:30


Chemistry, 14.10.2019 18:30


History, 14.10.2019 18:30


Biology, 14.10.2019 18:30

English, 14.10.2019 18:30


Social Studies, 14.10.2019 18:30

Social Studies, 14.10.2019 18:30

Biology, 14.10.2019 18:30


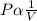 This means , PV = k (constant)Therefore; P₁V₁ = P₂V₂Rearranging the formula, we can get the new pressure, P₂
This means , PV = k (constant)Therefore; P₁V₁ = P₂V₂Rearranging the formula, we can get the new pressure, P₂


