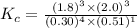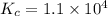Chemistry, 06.12.2019 06:31 btcastongia
Part a what is the numerical value of kc for the following reaction if the equilibrium mixture contains 0.51 m c3h6o, 0.30 m o2, 1.8 m co2, and 2.0 m h2o? c3h6o(g)+4o2(g)⇌3co2(g)+3h2o(g)
a) 2.4 × 101b) 1.1 × 104c) 8.9 × 10-5d) 4.3 × 10-2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

You know the right answer?
Part a what is the numerical value of kc for the following reaction if the equilibrium mixture conta...
Questions

Mathematics, 14.06.2021 02:40

Social Studies, 14.06.2021 02:40

Mathematics, 14.06.2021 02:40



English, 14.06.2021 02:40


Mathematics, 14.06.2021 02:40


English, 14.06.2021 02:40


Mathematics, 14.06.2021 02:40


Mathematics, 14.06.2021 02:40

Mathematics, 14.06.2021 02:40


Mathematics, 14.06.2021 02:40


Mathematics, 14.06.2021 02:40

Mathematics, 14.06.2021 02:40


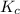 .
.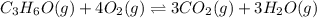
![K_c=\frac{[CO_2]^3\times [H_2O]^3}{[O_2]^4\times [C_3H_6O]^1}](/tpl/images/0406/0681/8cf44.png)