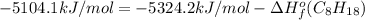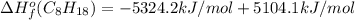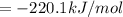Chemistry, 06.12.2019 05:31 breannamiller0822
Or a particular isomer of c 8 h 18 , the combustion reaction produces 5104.1 kj of heat per mole of c 8 h 18 ( g ) consumed, under standard conditions. c 8 h 18 ( g ) + 25 2 o 2 ( g ) ⟶ 8 co 2 ( g ) + 9 h 2 o ( g ) δ h ∘ rxn = − 5104.1 kj / mol what is the standard enthalpy of formation of this isomer of c 8 h 18 ( g ) ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Or a particular isomer of c 8 h 18 , the combustion reaction produces 5104.1 kj of heat per mole of...
Questions


Mathematics, 04.06.2021 02:50


Mathematics, 04.06.2021 02:50

Mathematics, 04.06.2021 02:50

World Languages, 04.06.2021 02:50


World Languages, 04.06.2021 02:50


Mathematics, 04.06.2021 02:50

Physics, 04.06.2021 02:50



Mathematics, 04.06.2021 02:50


Mathematics, 04.06.2021 02:50

Social Studies, 04.06.2021 02:50



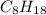 is -220.1 kJ/mol.
is -220.1 kJ/mol.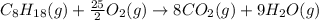
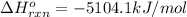
![\Delta H^{o}_{rxn}=[8\Delta H^{o}_{f}(CO_{2}) +9\Delta H^{o}_{f}(H_{2}O)]-[\Delta H^{o}_{f}(C_{8}H_{18})+ \frac{25}{2}\Delta H^{o}_{f}(O_{2})]](/tpl/images/0405/9197/1d3a2.png)
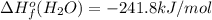
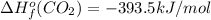
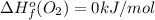
![-5104.1kJ/mol=[8(-393.5)+9(-241.8)kJ/mol]-[\Delta H^{o}_{f}(C_{8}H_{18})+ \frac{25}{2}(0)kJ/mol]](/tpl/images/0405/9197/edc02.png)