
Chemistry, 06.12.2019 05:31 haitiindianari
Consider the reaction 4 ph3(g) → p4(g) + 6 h2(g). if, in a certain experiment, over a specific time period, 0.0049 mole of ph3 is consumed in a 2.4-l container during each second of the reaction, what are the rates of production of p4 and h2 in this experiment?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Consider the reaction 4 ph3(g) → p4(g) + 6 h2(g). if, in a certain experiment, over a specific time...
Questions


Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01



Mathematics, 03.08.2020 14:01

English, 03.08.2020 14:01




Mathematics, 03.08.2020 14:01

Biology, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

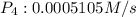
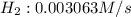
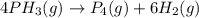
![R=-\frac{1}{4}\frac{d[PH_3]}{dt}=\frac{1}{1}\frac{d[P_4]}{dt}=\frac{1}{6}\frac{d[H_2]}{dt}](/tpl/images/0405/9326/1b2ce.png)
![-\frac{d[PH_3]}{dt}](/tpl/images/0405/9326/0026c.png)
![\frac{[PH_3]}{1 s}](/tpl/images/0405/9326/00de6.png)

![-\frac{d[PH_3]}{dt}=0.002042 M/s](/tpl/images/0405/9326/a6182.png)
![R = -\frac{1}{4}\frac{d[PH_3]}{dt}=\frac{1}{4}\times 0.002042 M/s=0.0005105 M/s](/tpl/images/0405/9326/7d03f.png)
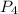 :
:![R=\frac{1}{1}\frac{d[P_4]}{dt}](/tpl/images/0405/9326/34938.png)
![\frac{d[P_4]}{dt}=\frac{1}{1}\times 0.0005105 M/s=0.0005105 M/s](/tpl/images/0405/9326/4315e.png)
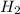 :
:![R=\frac{1}{6}\frac{d[P_4]}{dt}](/tpl/images/0405/9326/3100b.png)
![\frac{d[H_2]}{dt}=\frac{6}{1}\times 0.0005105 M/s=0.003063 M/s](/tpl/images/0405/9326/b8130.png)



