Chemistry, 06.12.2019 05:31 AaronMicrosoft15
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the corresponding δg∘ and e∘cel under standard conditions.
δg∘ =
e∘cell =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the co...
Questions

Mathematics, 08.03.2021 23:40





Mathematics, 08.03.2021 23:40

Chemistry, 08.03.2021 23:40

Spanish, 08.03.2021 23:40




Arts, 08.03.2021 23:40

Arts, 08.03.2021 23:40

English, 08.03.2021 23:40


Mathematics, 08.03.2021 23:40

Mathematics, 08.03.2021 23:40

History, 08.03.2021 23:40


Mathematics, 08.03.2021 23:40

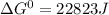
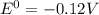
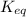
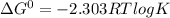
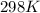
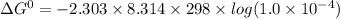
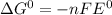
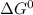 = gibbs free energy = 22823J
= gibbs free energy = 22823J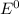 = standard emf
= standard emf