
Chemistry, 06.12.2019 05:31 DisneyyKayy
Calculate δh∘ in kilojoules for the reaction of acetylene (c2h2) (δh∘f=227.4kj/mol) with o2 to yield carbon dioxide (co2) (δh∘f=−393.5 kj/mol) and h2o(g) (δh∘f=−241.8kj/mol), a reaction which is supplied by the industrial gases industry for oxyacetylene gas welding and cutting due to the high temperature of the flame.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1


Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Calculate δh∘ in kilojoules for the reaction of acetylene (c2h2) (δh∘f=227.4kj/mol) with o2 to yield...
Questions


Business, 12.07.2019 20:00



History, 12.07.2019 20:00

Physics, 12.07.2019 20:00

Social Studies, 12.07.2019 20:00

History, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00

History, 12.07.2019 20:00


History, 12.07.2019 20:00

History, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00


History, 12.07.2019 20:00

Mathematics, 12.07.2019 20:00

Biology, 12.07.2019 20:00

Biology, 12.07.2019 20:00

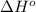 for the reaction is, -2512.4 kJ
for the reaction is, -2512.4 kJ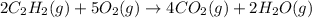
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_2H_2(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0405/9362/62f19.png)
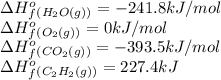
![\Delta H^o_{rxn}=[(4\times (-393.5))+(2\times (-241.8))]-[(2\times (227.4)+(5\times (0))]\\\\\Delta H^o_{rxn}=-2512.4kJ](/tpl/images/0405/9362/361f8.png)


