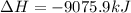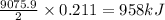Chemistry, 06.12.2019 05:31 lindseylewis313
B5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released when 0.211 mol of b5h9 reacts with excess oxygen where the products are b2h3(s) and h2o(l). the standard enthalpy of formation of b5h9(l) is 73.2 kj/mol, the standard enthalpy of formation of b2h3(s) is -1272 kj/mol and that of h2o(l) is -285.4 kj/mol. express your answer in kj.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
B5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released wh...
Questions


Mathematics, 29.08.2019 03:10

Mathematics, 29.08.2019 03:10

Mathematics, 29.08.2019 03:10

History, 29.08.2019 03:10

Social Studies, 29.08.2019 03:10


Mathematics, 29.08.2019 03:10



Mathematics, 29.08.2019 03:10

Mathematics, 29.08.2019 03:10

Computers and Technology, 29.08.2019 03:10







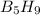 reacts with excess oxygen.
reacts with excess oxygen.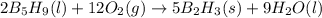
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0405/9477/76c37.png)
![\Delta H=[(n_{B_2H_3}\times \Delta H_{B_2H_3})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{B_5H_9}\times \Delta H_{B_5H_9})]](/tpl/images/0405/9477/bc69c.png)
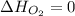 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(5\times -1272)+(9\times -285.5]-[(12\times 0)+(2\times 73.2)]](/tpl/images/0405/9477/56397.png)