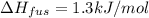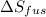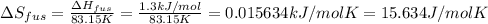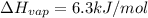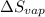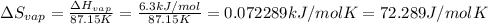Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1


Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
The molar heats of fusion and vaporization of argon are 1.3 kj/mol and 6.3 kj/mol respectively, and...
Questions




Mathematics, 17.10.2019 18:30


English, 17.10.2019 18:30

Computers and Technology, 17.10.2019 18:30

History, 17.10.2019 18:30

Biology, 17.10.2019 18:30




History, 17.10.2019 18:30






History, 17.10.2019 18:30