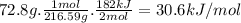Chemistry, 06.12.2019 04:31 phillipsk5480
Achemist measures the energy change ? h during the following reaction: 2hgo (s) ? 2hg (l) +o2 (g) =? h182.kj use the information to answer the following questions. this reaction
a. endothermic.
b. exothermic.
suppose 72.8g of hgo react. will any heat be released or absorbed?
a. yes, absorbed.
b. yes, released.
c. no.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

You know the right answer?
Achemist measures the energy change ? h during the following reaction: 2hgo (s) ? 2hg (l) +o2 (g) =...
Questions





History, 12.07.2021 18:40




Mathematics, 12.07.2021 18:40


Biology, 12.07.2021 18:40

Mathematics, 12.07.2021 18:40

English, 12.07.2021 18:40



Mathematics, 12.07.2021 18:40