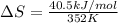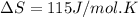Chemistry, 06.12.2019 04:31 kellysmith45
Calculate the standard entropy of vaporization of ethanol at its boiling point, 352 k. the standard molar enthalpy of vaporization of ethanol at its boiling point is 40.5 kj/mol.
a. +40.5 j/mol k
b. +115 j/mol k
c. -40.5 j/mol k
d. -115 j/mol k

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1
You know the right answer?
Calculate the standard entropy of vaporization of ethanol at its boiling point, 352 k. the standard...
Questions



History, 05.11.2020 01:00


English, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

Arts, 05.11.2020 01:00

Physics, 05.11.2020 01:00



Mathematics, 05.11.2020 01:00

Chemistry, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

English, 05.11.2020 01:00


Mathematics, 05.11.2020 01:00


Mathematics, 05.11.2020 01:00

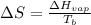
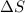 = change in entropy
= change in entropy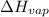 = change in enthalpy of vaporization = 40.5 kJ/mol
= change in enthalpy of vaporization = 40.5 kJ/mol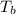 = boiling point temperature = 352 K
= boiling point temperature = 352 K