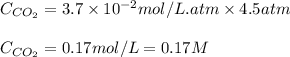Chemistry, 06.12.2019 04:31 Officaljazz18
Enter your answer in the provided box. the partial pressure of co2 gas above the liquid in a bottle of champagne at 20°c is 4.5 atm. what is the solubility of co2 in champagne? assume the henry's law constant is the same for champagne as it is for water: at 20°c, kh = 3.7 × 10−2 mol/(l·atm).

Answers: 3


Another question on Chemistry




You know the right answer?
Enter your answer in the provided box. the partial pressure of co2 gas above the liquid in a bottle...
Questions



Biology, 27.03.2020 23:20


World Languages, 27.03.2020 23:20


Social Studies, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20

History, 27.03.2020 23:20




Mathematics, 27.03.2020 23:20

History, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20


English, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20

Biology, 27.03.2020 23:20

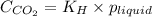
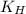 = Henry's constant =
= Henry's constant = 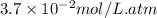
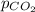 = partial pressure of carbonated drink = 4.5 atm
= partial pressure of carbonated drink = 4.5 atm