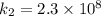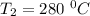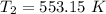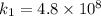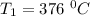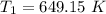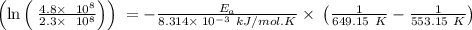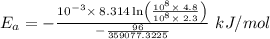Chemistry, 06.12.2019 02:31 jessicamcgoldri5625
The rate constant k for a certain reaction is measured at two different temperatures temperature 376.0 °c 4.8 x 108 280.0 °c 2.3 x 10 8 assuming the rate constant obeys the arrhenius equation, calculate the activation energy e for this reaction. round your answer to 2 significant digits. kj x10 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
The rate constant k for a certain reaction is measured at two different temperatures temperature 376...
Questions

Mathematics, 21.05.2021 17:30



English, 21.05.2021 17:30

Mathematics, 21.05.2021 17:30

Mathematics, 21.05.2021 17:30



Mathematics, 21.05.2021 17:30


Mathematics, 21.05.2021 17:30



Mathematics, 21.05.2021 17:30


Mathematics, 21.05.2021 17:30

Mathematics, 21.05.2021 17:30

Social Studies, 21.05.2021 17:30


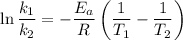
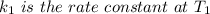
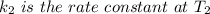
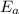 is the activation energy
is the activation energy