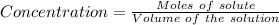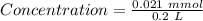Chemistry, 06.12.2019 02:31 bvaughn4152
Achemist prepares a solution of nickel(ii) chloride nicl2 by measuring out 21.μmol of nickel(ii) chloride into a 200.ml volumetric flask and filling the flask to the mark with water. calculate the concentration in /mmoll of the chemist's nickel(ii) chloride solution. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
Achemist prepares a solution of nickel(ii) chloride nicl2 by measuring out 21.μmol of nickel(ii) chl...
Questions


Mathematics, 06.03.2020 19:30


History, 06.03.2020 19:30




Mathematics, 06.03.2020 19:31






Computers and Technology, 06.03.2020 19:31


History, 06.03.2020 19:31

Arts, 06.03.2020 19:31

Mathematics, 06.03.2020 19:31


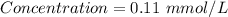
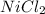 = 21 μmol
= 21 μmol