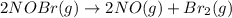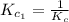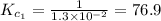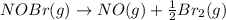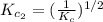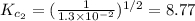Chemistry, 06.12.2019 02:31 taylorb9893
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at this temperature does the equilibrium favor no and br2, or does it favor nobr? calculate kc for 2nobr(g)⥫⥬==2no(g)+br2(g). calculate kc for nobr(g)⥫⥬==no(g)+12br2(g).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at thi...
Questions

Advanced Placement (AP), 01.09.2019 22:00

History, 01.09.2019 22:00


English, 01.09.2019 22:00


Chemistry, 01.09.2019 22:00


Mathematics, 01.09.2019 22:00









History, 01.09.2019 22:00

History, 01.09.2019 22:00

Geography, 01.09.2019 22:00

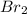 .
.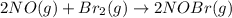
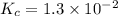 .
.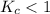 that means equilibrium lies to the left side. Thus, the equilibrium favors NO and
that means equilibrium lies to the left side. Thus, the equilibrium favors NO and