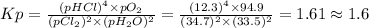Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2(g) + 2h2o(g) → 4hcl(g) + o2(g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound pressure at equilibrium cl2 34.7atm h2o 33.5atm hcl 12.3atm o2 94.9atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2(g) + 2h2o(g) → 4hcl(...
Questions



Mathematics, 25.03.2021 22:20

History, 25.03.2021 22:20



Mathematics, 25.03.2021 22:20

Mathematics, 25.03.2021 22:20

Computers and Technology, 25.03.2021 22:20

Social Studies, 25.03.2021 22:20



Computers and Technology, 25.03.2021 22:20

Mathematics, 25.03.2021 22:20




Arts, 25.03.2021 22:20


Chemistry, 25.03.2021 22:20