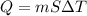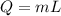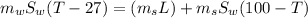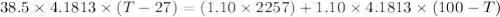Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
If 1.10 g of steam at 100.0 °c condenses into 38.5 g of water, initially at 27.0 °c, in an insulated...
Questions

Physics, 11.03.2021 04:00

English, 11.03.2021 04:00

Chemistry, 11.03.2021 04:00

Mathematics, 11.03.2021 04:00

History, 11.03.2021 04:00

English, 11.03.2021 04:00


Advanced Placement (AP), 11.03.2021 04:00



English, 11.03.2021 04:00

Biology, 11.03.2021 04:00

Chemistry, 11.03.2021 04:00






Physics, 11.03.2021 04:00