
Chemistry, 05.12.2019 23:31 maljoh8249
Enter your answer in the provided box. an industrial chemist studying bleaching and sterilizing prepares a hypochlorite buffer using 0.450 m hclo and 0.450 m naclo. (ka for hclo = 2.9 × 10−8) find the ph of 1.00 l of the solution after 0.040 mol of naoh has been added.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3


Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
Enter your answer in the provided box. an industrial chemist studying bleaching and sterilizing prep...
Questions





Mathematics, 12.12.2020 16:00


English, 12.12.2020 16:00



English, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Computers and Technology, 12.12.2020 16:00

History, 12.12.2020 16:00


Spanish, 12.12.2020 16:00




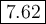
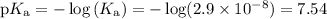
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 7.54 +\log \left(\dfrac{0.450}{0.450}\right )\\\\& = & 7.54 + \log1.00 \\ & = & 7.54 + 0.00\\& = & 7.54\\\end{array}](/tpl/images/0405/3467/7ad02.png)

![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 7.54 +\log \left(\dfrac{490}{410}\right )\\\\& = & 7.54 + \log1.195 \\& = & 7.54 +0.0774\\& = & \mathbf{7.62}\\\end{array}\\\text{The new pH is $\large \boxed{\textbf{7.62}}$}](/tpl/images/0405/3467/ab2c6.png)



