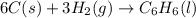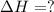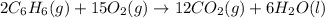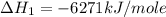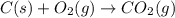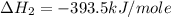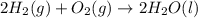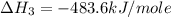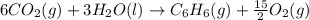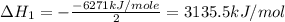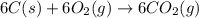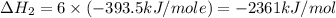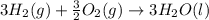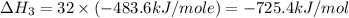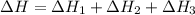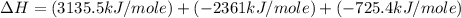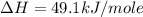Chemistry, 05.12.2019 23:31 carlinryan
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g) → co₂ (g) ∆h° = -393.5 kj/mol 2 h₂ (g) + o₂ (g) → 2 h₂o (g) ∆h° = -483.6 kj/mol determine the enthalpy for the reaction 6 c (s) + 3 h₂ (g) → c₆h₆ (l).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g)...
Questions



Mathematics, 18.02.2021 23:50

Mathematics, 18.02.2021 23:50


Spanish, 18.02.2021 23:50

Law, 18.02.2021 23:50

Mathematics, 18.02.2021 23:50

Mathematics, 18.02.2021 23:50

Biology, 18.02.2021 23:50

Mathematics, 18.02.2021 23:50





Mathematics, 18.02.2021 23:50


Mathematics, 18.02.2021 23:50


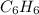 will be,
will be,