
Chemistry, 05.12.2019 22:31 cuthbertson157
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of carbon dioxide at 20.0 â°c and 791 mmhg. calculate the percent by mass of calcium carbonate in the sample.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of c...
Questions









SAT, 30.11.2021 01:40




Biology, 30.11.2021 01:40

Computers and Technology, 30.11.2021 01:40



Mathematics, 30.11.2021 01:40

SAT, 30.11.2021 01:40


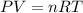
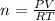
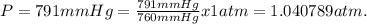
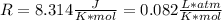
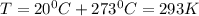
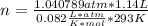
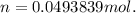
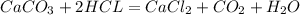
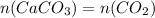
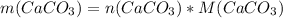
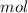 * 100
* 100 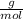 = 4.93839
= 4.93839 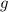
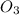 =
= 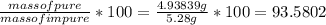 .
.


