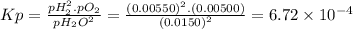Chemistry, 05.12.2019 22:31 jacxirylopez
The elementary reaction 2 h 2 o ( g ) − ⇀ ↽ − 2 h 2 ( g ) + o 2 ( g ) proceeds at a certain temperature until the partial pressures of h 2 o , h 2 , and o 2 reach 0.0150 bar , 0.00550 bar , and 0.00500 bar respectively. what is the value of the equilibrium constant at this temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1



Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
The elementary reaction 2 h 2 o ( g ) − ⇀ ↽ − 2 h 2 ( g ) + o 2 ( g ) proceeds at a certain temperat...
Questions




English, 21.04.2020 04:20


History, 21.04.2020 04:20

Advanced Placement (AP), 21.04.2020 04:20

Mathematics, 21.04.2020 04:20

Geography, 21.04.2020 04:20




Mathematics, 21.04.2020 04:20


Mathematics, 21.04.2020 04:20

English, 21.04.2020 04:20

Chemistry, 21.04.2020 04:20