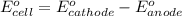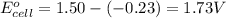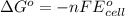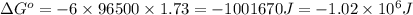Chemistry, 05.12.2019 22:31 angel0203wilcox
Calculate the standard free-energy change for the reaction at 25 ∘ c. 25 ∘c. refer to the list of standard reduction potentials. 2 au 3 + (aq) + 3 ni (s) − ⇀ ↽ − 2 au (s) + 3 ni 2 + (aq) 2au3+(aq)+3ni(s)↽−−⇀2au(s)+3ni2+(aq ) δ g ∘ = δg∘=

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
Calculate the standard free-energy change for the reaction at 25 ∘ c. 25 ∘c. refer to the list of st...
Questions




History, 12.08.2020 05:01






English, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01


English, 12.08.2020 05:01

Chemistry, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

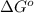 for the given reaction is
for the given reaction is 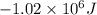
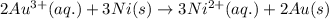
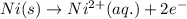 ( × 3)
( × 3)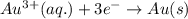 ( × 2)
( × 2)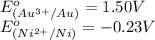
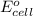 of the reaction, we use the equation:
of the reaction, we use the equation: