
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction. ag+(aq)+e−→ag(s) part a silver can be electroplated at the cathode of an electrolysis cell according to the following half-reaction: ag+(aq)+e−→ag(s) what mass of silver plates onto the cathode when a current of 8.5 a flows through the cell for 68 min ?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction. ag+(aq)+e−→...
Questions



Mathematics, 30.07.2020 04:01




Mathematics, 30.07.2020 04:01





Biology, 30.07.2020 04:01



Mathematics, 30.07.2020 04:01





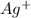 +e− ==> Ag(s)
+e− ==> Ag(s)


