
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an empty 5.8-l bulb, then filled it with the gas at 1.00 atm and 21.0 ∘c and weighed it again. the difference in mass was 6.7 g. identify the gas. express your answer as a chemical formula.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an...
Questions






Mathematics, 13.11.2020 05:40

Social Studies, 13.11.2020 05:40


Mathematics, 13.11.2020 05:40

Mathematics, 13.11.2020 05:40

Mathematics, 13.11.2020 05:40

History, 13.11.2020 05:40

Health, 13.11.2020 05:40


Mathematics, 13.11.2020 05:40


Arts, 13.11.2020 05:40


History, 13.11.2020 05:40

Mathematics, 13.11.2020 05:40

 = (21 + 273) K = 294 K, mass = 6.7 g
= (21 + 273) K = 294 K, mass = 6.7 g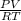



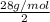
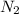 .
.


