
Chemistry, 18.12.2019 03:31 dalechloe5596
Which of the following processes would you predict to have an increase in entropy?
condensation of water
freezing water
melting ice
deposition of co2 (changing from a gas to solid)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 16:30
The form of energy that can move from place to place across the universe is (answer) on earth, the main source of this energy is (2nd answer) first blank; a) gravitational potential energy b) radiant energy c)thermal energy d) vibrational energy second blank; a)lightning b) the moon c) the sun d) the wind
Answers: 1
You know the right answer?
Which of the following processes would you predict to have an increase in entropy?
cond...
cond...
Questions


Mathematics, 03.08.2020 14:01


Geography, 03.08.2020 14:01




Mathematics, 03.08.2020 14:01



Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Biology, 03.08.2020 14:01




Mathematics, 03.08.2020 14:01

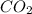 : deposition process where gaseous state changes to solid state by escaping liquid state, thus decreasing randomness and decreasing entropy.
: deposition process where gaseous state changes to solid state by escaping liquid state, thus decreasing randomness and decreasing entropy.

