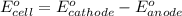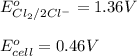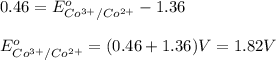Chemistry, 05.12.2019 18:31 eyeneedalife
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell = 0.46 v. given e° = 1.36 v for the reaction cl2(g) + 2e– → 2cl–(aq), calculate the standard reduction potential for the following the half reaction at 25°c: co3+ + e– → co2+

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell...
Questions



History, 05.06.2021 05:20


Arts, 05.06.2021 05:20



Mathematics, 05.06.2021 05:20



Chemistry, 05.06.2021 05:20


History, 05.06.2021 05:20






Mathematics, 05.06.2021 05:20

Biology, 05.06.2021 05:20

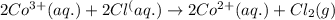
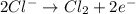
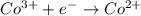
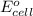 of the reaction, we use the equation:
of the reaction, we use the equation: