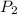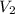Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1


Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Afixed amount of gas at 25.0°c occupies a volume of 10.0 l when the pressure is 751 torr. use boyle'...
Questions


Mathematics, 14.12.2020 19:30




Mathematics, 14.12.2020 19:30



Social Studies, 14.12.2020 19:30


Chemistry, 14.12.2020 19:30

Mathematics, 14.12.2020 19:30

Computers and Technology, 14.12.2020 19:30

English, 14.12.2020 19:30



Mathematics, 14.12.2020 19:30

English, 14.12.2020 19:30

English, 14.12.2020 19:30

Mathematics, 14.12.2020 19:30

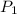
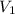 =
=