when ethane c2h6) burns, it produces carbon dioxide and water:
2c3h5(0) +702(g) → 4co2(g) + 6...

Chemistry, 05.12.2019 09:31 wyattdawes45
when ethane c2h6) burns, it produces carbon dioxide and water:
2c3h5(0) +702(g) → 4co2(g) + 6h2011)
how many liters of carbon dioxide will be produced when 269 l of ethane are
burned? (one mole of any gas occupies 22.4 l under certain conditions of
temperature and pressure. assume those conditions for this question.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Questions

Mathematics, 20.05.2020 05:00

Biology, 20.05.2020 05:00

Mathematics, 20.05.2020 05:00




Mathematics, 20.05.2020 05:00


Mathematics, 20.05.2020 05:00

History, 20.05.2020 05:00


Mathematics, 20.05.2020 05:00


Mathematics, 20.05.2020 05:00


Arts, 20.05.2020 05:00

Mathematics, 20.05.2020 05:00

Mathematics, 20.05.2020 05:00


Mathematics, 20.05.2020 05:00

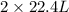 of ethane gives
of ethane gives 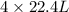 of CO₂.
of CO₂.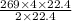
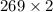 L
L

