
Chemistry, 05.12.2019 05:31 Jcausey4477
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs to be done twice-once fro co2 and once for h20.
b) if 0.647 mole of oxygen in used in the burning of propane, how many moles of each of h2o are produced? how many moles of c3h8 are consumed?
the balanced chemical reaction:
1 c3 + 5 o2 = 3co2 + 4 h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs t...
Questions




History, 18.11.2019 09:31


Biology, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31



SAT, 18.11.2019 09:31



Physics, 18.11.2019 09:31

Mathematics, 18.11.2019 09:31

Chemistry, 18.11.2019 09:31


Mathematics, 18.11.2019 09:31

History, 18.11.2019 09:31


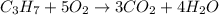
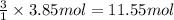 of carbon dioxide gas.
of carbon dioxide gas.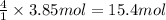 of water .
of water .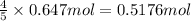 of water.
of water.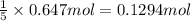 of propane.
of propane.

