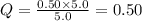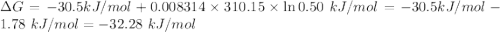Chemistry, 05.12.2019 03:31 chevysilverado3464
For which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.50 mm, and [hpo42–] = 5.0 mm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1

Chemistry, 23.06.2019 15:30
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3+(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1
You know the right answer?
For which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological...
Questions




Health, 01.07.2019 13:00

Mathematics, 01.07.2019 13:00

English, 01.07.2019 13:00


History, 01.07.2019 13:00

Advanced Placement (AP), 01.07.2019 13:00




Mathematics, 01.07.2019 13:00


History, 01.07.2019 13:00

English, 01.07.2019 13:00


History, 01.07.2019 13:00


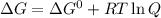
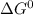 standard Gibbs energy
standard Gibbs energy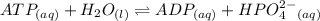
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0403/8875/ccdf0.png)
![[ATP]=5.0 mM](/tpl/images/0403/8875/1ddd2.png)
![[ADP]=0.50 mM](/tpl/images/0403/8875/91d08.png)
![[HPO_4^{2-}]=5.0 mM](/tpl/images/0403/8875/ff97d.png)