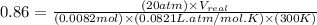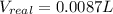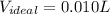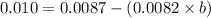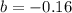Chemistry, 05.12.2019 02:31 amanda1717
At 300 k and 20 atm, the compression factor of a gas is 0.86. calculate (a) the volume occupied by 8.2 mmol of the gas under these conditions and (b) an approximate value of the second virial coefficient b at 300 k. (20 pts)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
At 300 k and 20 atm, the compression factor of a gas is 0.86. calculate (a) the volume occupied by 8...
Questions

Mathematics, 16.12.2020 20:30

History, 16.12.2020 20:30




Mathematics, 16.12.2020 20:30



Mathematics, 16.12.2020 20:30



Arts, 16.12.2020 20:30

Chemistry, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Biology, 16.12.2020 20:30



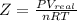
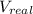 = volume of gas = ?
= volume of gas = ?