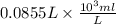Chemistry, 04.12.2019 21:31 magicalforlife
You have 0.500 l of an 0.250 m acetate buffer solution (i. e. [hc₂h₃o₂] + [c₂h₃o₂⁻] = 0.250 m) at ph 3.50. how many ml of 1.000 m naoh must you add in order to change the ph to 5.25? acetic acid has a pka of 4.74.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
You have 0.500 l of an 0.250 m acetate buffer solution (i. e. [hc₂h₃o₂] + [c₂h₃o₂⁻] = 0.250 m) at ph...
Questions




English, 25.05.2020 08:58

Mathematics, 25.05.2020 08:58

Mathematics, 25.05.2020 08:58

Mathematics, 25.05.2020 08:58


Mathematics, 25.05.2020 08:58

Mathematics, 25.05.2020 08:58


History, 25.05.2020 08:58

Mathematics, 25.05.2020 08:58

History, 25.05.2020 08:58


Mathematics, 25.05.2020 08:58

English, 25.05.2020 08:58



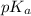 is as follows.
is as follows.![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]}](/tpl/images/0403/2718/85431.png)
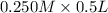
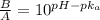
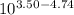
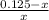 = 0.0575
= 0.0575![log \frac{[B]}{[A]}](/tpl/images/0403/2718/93cf8.png)
![log \frac{[B]}{[A]} = 10^{5.25 - 4.74}](/tpl/images/0403/2718/76315.png)
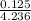
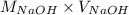 = (0.118 - 0.0295) moles
= (0.118 - 0.0295) moles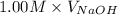 = 0.0885 moles
= 0.0885 moles