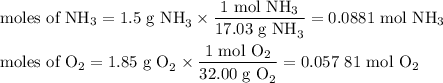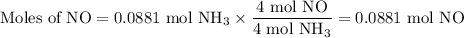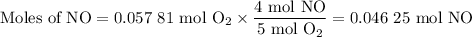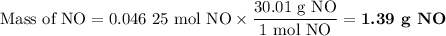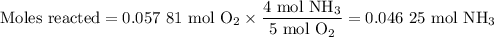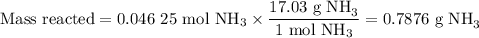no:

Chemistry, 04.12.2019 19:31 ErickP1686
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
nh3 + o2 + no + h2o
balanced: 4nh3 +502 uno+ 6h2o
a.) how many grams of no form when 1.5g of nh3 reacts with 1.85g of o2? b.) which
reactant is the limiting reactant and which one is the excess reactant? c.) how much of
the excess reactant remains after the limiting reactant is completely consumed?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
no:
Questions




Mathematics, 26.10.2019 21:43

Biology, 26.10.2019 21:43

Mathematics, 26.10.2019 21:43


Health, 26.10.2019 21:43


Social Studies, 26.10.2019 21:43


Geography, 26.10.2019 21:43


Mathematics, 26.10.2019 21:43


Geography, 26.10.2019 21:43

Mathematics, 26.10.2019 21:43



Geography, 26.10.2019 21:43