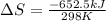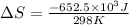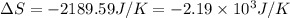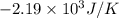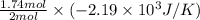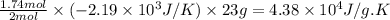Chemistry, 04.12.2019 07:31 pheonixhowls
Consider the reaction 2na(s) + 2h2o(l)2naoh(aq) + h2(g) using standard thermodynamic data at 298k, calculate the entropy change for the surroundings when 1.74 moles of na(s) react at standard conditions. s°surroundings = j/k g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1
You know the right answer?
Consider the reaction 2na(s) + 2h2o(l)2naoh(aq) + h2(g) using standard thermodynamic data at 298k, c...
Questions

Mathematics, 11.10.2019 22:30

History, 11.10.2019 22:30

Mathematics, 11.10.2019 22:30

Mathematics, 11.10.2019 22:30

Mathematics, 11.10.2019 22:30

English, 11.10.2019 22:30





Mathematics, 11.10.2019 22:30


Mathematics, 11.10.2019 22:30






Biology, 11.10.2019 22:30

Mathematics, 11.10.2019 22:30

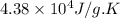
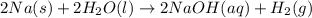
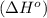 .
.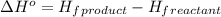
![\Delta H^o=[n_{NaOH}\times \Delta H_f^0_{(NaOH)}+n_{H_2}\times \Delta H_f^0_{(H_2)}]-[n_{Na}\times \Delta H_f^0_{(Na)+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]](/tpl/images/0402/5848/95f1f.png)
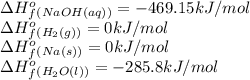
![\Delta H^o_{rxn}=[(2\times -469.15)+(1\times 0)]-[(2\times 0)+(2\times -285.8)]=-652.5kJ](/tpl/images/0402/5848/c5dc6.png)
 .
.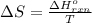
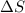 = change in entropy
= change in entropy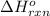 = change in enthalpy = -652.5 kJ
= change in enthalpy = -652.5 kJ