
Chemistry, 04.12.2019 05:31 AngelH2650
Use the following information on cr to determine the amounts of heat for the three heating steps required to convert 126.3 g of solid cr at 1760°c into liquid cr at 2060°c. mp = 1860°c bp = 2672°c enter in kj. useful data: \delta hδ hfus = 20.5 kj/mol; \delta hδ hvap = 339 kj/mol; c(solid) 44.8 j/g°c; c(liquid) = 0.94 j/g°c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Use the following information on cr to determine the amounts of heat for the three heating steps req...
Questions

History, 29.10.2019 03:31

Mathematics, 29.10.2019 03:31

Mathematics, 29.10.2019 03:31



English, 29.10.2019 03:31



Mathematics, 29.10.2019 03:31

Mathematics, 29.10.2019 03:31



Mathematics, 29.10.2019 03:31


Mathematics, 29.10.2019 03:31

Mathematics, 29.10.2019 03:31


Mathematics, 29.10.2019 03:31


Mathematics, 29.10.2019 03:31

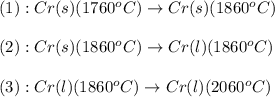
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0402/3904/5cd06.png)
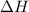 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?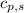 = specific heat of solid Cr =
= specific heat of solid Cr = 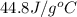
 = specific heat of liquid Cr =
= specific heat of liquid Cr = 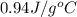
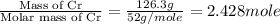
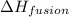 = enthalpy change for fusion = 20.5 KJ/mole = 20500 J/mole
= enthalpy change for fusion = 20.5 KJ/mole = 20500 J/mole![\Delta H=[126.3g\times 44.8J/g^oC\times (1860-(1760))^oC]+2.428mole\times 20500J/mole+[126.3g\times 0.94J/g^oC\times (2060-1860)^oC]](/tpl/images/0402/3904/d29ca.png)
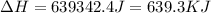 (1 KJ = 1000 J)
(1 KJ = 1000 J)

