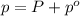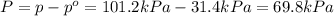Chemistry, 04.12.2019 04:31 alannaamarriee
Last week you reacted magnesium with a hydrochloric acid aqueous solution and hydrogen gas was produced. let's say that you collected the gas given off by the reaction and measured it's pressure as 101.2 kpa. if the vapor pressure of water is 31.4 kpa at this temperature, then what is the pressure of the hydrogen gas?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2
You know the right answer?
Last week you reacted magnesium with a hydrochloric acid aqueous solution and hydrogen gas was produ...
Questions

English, 04.02.2021 21:20


Spanish, 04.02.2021 21:20



Mathematics, 04.02.2021 21:20

History, 04.02.2021 21:20

Mathematics, 04.02.2021 21:20



Mathematics, 04.02.2021 21:20

Physics, 04.02.2021 21:20





Spanish, 04.02.2021 21:20



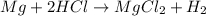
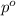 = 31.4 kilo Pascals
= 31.4 kilo Pascals