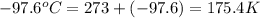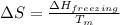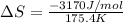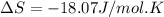Chemistry, 04.12.2019 04:31 cutebabyolivia
What is the entropy change for the freezing process of 1 mole of liquid methanol at its freezing temperature (–97.6˚c) and 1 atm? report your answer two points past the decimal with the unit j/molk. ∆h˚fus = 3.17 kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
What is the entropy change for the freezing process of 1 mole of liquid methanol at its freezing tem...
Questions


Arts, 18.12.2020 21:20


Mathematics, 18.12.2020 21:20


Mathematics, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20




History, 18.12.2020 21:20



Mathematics, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20

Mathematics, 18.12.2020 21:20



Biology, 18.12.2020 21:20

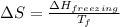
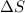 = change in entropy
= change in entropy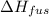 = change in enthalpy of fusion = 3.17 kJ/mol
= change in enthalpy of fusion = 3.17 kJ/mol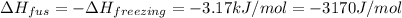
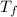 = freezing point temperature =
= freezing point temperature =