
Chemistry, 04.12.2019 02:31 dancer2814
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (r = 2.179 x 10-18 j r = 1.096776 x 10^7 m-1) a. 205.1 nm b. 384.6 nm c. 683.8 nm d. 1282 nm e. > 1500 nm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infra...
Questions

History, 21.08.2019 01:30





Mathematics, 21.08.2019 01:30

Physics, 21.08.2019 01:30


Mathematics, 21.08.2019 01:30

Mathematics, 21.08.2019 01:30

English, 21.08.2019 01:30

Mathematics, 21.08.2019 01:30








Social Studies, 21.08.2019 01:30

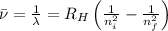
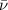 = Wave number
= Wave number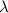 = Wavelength of radiation
= Wavelength of radiation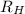 = Rydberg's Constant
= Rydberg's Constant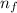 = Higher energy level
= Higher energy level 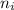 = Lower energy level
= Lower energy level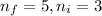
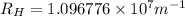
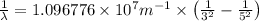
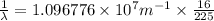
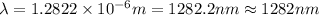
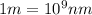 )
)

