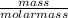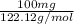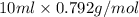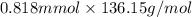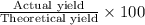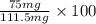Chemistry, 04.12.2019 00:31 Aydenj9613
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric acid to produce methyl benzoate. write a balance chemical equation for this reaction. determine the limiting reagent and calculate a theoretical yield of both the ester and water. if you isolate 75 mg of methyl benzoate, what is the actual yield of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

You know the right answer?
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric a...
Questions


History, 29.01.2020 00:55

Mathematics, 29.01.2020 00:55

History, 29.01.2020 00:55

History, 29.01.2020 00:55


Mathematics, 29.01.2020 00:55



Health, 29.01.2020 00:55


Mathematics, 29.01.2020 00:55


History, 29.01.2020 00:55


Chemistry, 29.01.2020 00:55


History, 29.01.2020 00:55


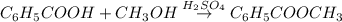
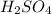 is very small so, that is catalytic amount of
is very small so, that is catalytic amount of