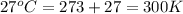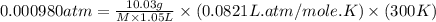Chemistry, 04.12.2019 00:31 zuleidysnegron
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at 27°c. the solution's osmotic pressure at 27°c is found to be 0.745 torr. calculate the molar mass of g/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

You know the right answer?
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at...
Questions

Mathematics, 18.11.2019 19:31

Biology, 18.11.2019 19:31

History, 18.11.2019 19:31

History, 18.11.2019 19:31

Chemistry, 18.11.2019 19:31



Mathematics, 18.11.2019 19:31


Mathematics, 18.11.2019 19:31





Mathematics, 18.11.2019 19:31





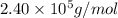
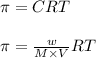
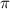 = osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)
= osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)