

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

You know the right answer?
The k b kb for an amine is 4.004 × 10 − 5 . 4.004×10−5. what percentage of the amine is protonated i...
Questions



Mathematics, 27.07.2019 21:40


History, 27.07.2019 21:40


History, 27.07.2019 21:40










Geography, 27.07.2019 21:50

English, 27.07.2019 21:50


Computers and Technology, 27.07.2019 21:50

![kb = \frac{[RNH_{4}^+][OH^-]}{[NH_{3}]}](/tpl/images/0401/7639/b9aa4.png)
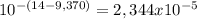 :
:![4,004x10^{-5} = \frac{[RNH_{4}^+][2,34x10^{-5}]}{[NH_{3}]}](/tpl/images/0401/7639/cd1a3.png)


