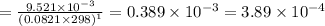Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Consider the following equilibrium.2 nobr(g)< => 2 no(g) + br2(g)if nitrosyl bromide, nobr, i...
Questions

Mathematics, 20.09.2020 17:01

English, 20.09.2020 17:01

Social Studies, 20.09.2020 17:01

Computers and Technology, 20.09.2020 17:01


History, 20.09.2020 17:01



Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Social Studies, 20.09.2020 17:01



Mathematics, 20.09.2020 17:01

History, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01

Chemistry, 20.09.2020 17:01


Computers and Technology, 20.09.2020 17:01

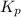 of the reaction is
of the reaction is 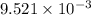 .
.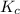 of the reaction is
of the reaction is 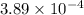 .
.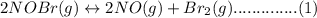
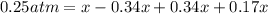
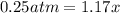
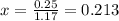
![[]P_{NOBr}]](/tpl/images/0401/8095/a6c6d.png) is 0.14 atm.
is 0.14 atm.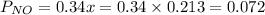
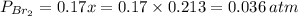
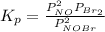
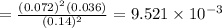
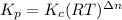
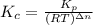
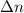 = number of moles of reactants - Number of moles of products
= number of moles of reactants - Number of moles of products