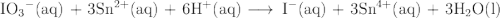Chemistry, 03.12.2019 22:31 Nakieapowell1276
Acidic solution in acidic solution, the iodate ion can be used to react with a number of metal ions. one such reaction is io3−(aq)+sn2+(aq)→i−(aq)+sn4+(aq) since this reaction takes place in acidic solution, h2o(l) and h+(aq) will be involved in the reaction. places for these species are indicated by the blanks in the following restatement of the equation: io3−(aq)+sn2+(aq)+ −−−→i−(aq)+sn4+(aq)+ −−−

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Acidic solution in acidic solution, the iodate ion can be used to react with a number of metal ions....
Questions




Mathematics, 25.07.2019 15:30





History, 25.07.2019 15:30



Physics, 25.07.2019 15:30



Mathematics, 25.07.2019 15:30


Physics, 25.07.2019 15:30

Arts, 25.07.2019 15:30


Computers and Technology, 25.07.2019 15:30