Chemistry, 03.12.2019 21:31 zaniathomasel
The combustion of pentane, c5h12, occurs via the reaction c5h12(g)+8o2(g)→5co2(g)+6h2o(g) with heat of formation values given by the following table: substance δh∘f (kj/mol) c5h12 (g) -119.9 co2(g) −393.5 h2o(g) −241.8 calculate the enthalpy for the combustion of pentane. express your answer to four significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
The combustion of pentane, c5h12, occurs via the reaction c5h12(g)+8o2(g)→5co2(g)+6h2o(g) with heat...
Questions



Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00


Biology, 06.03.2021 01:00


History, 06.03.2021 01:00


Mathematics, 06.03.2021 01:00


Mathematics, 06.03.2021 01:00





Mathematics, 06.03.2021 01:00


Mathematics, 06.03.2021 01:00

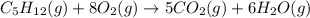
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0401/5602/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{CO_2}\times \Delta H_{CO_2})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_5H_{12}}\times \Delta H_{C_5H_{12}})]](/tpl/images/0401/5602/99eed.png)
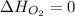 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(6\times -241.8)+(5\times -393.5)]-[(8\times 0)+(1\times -119.9)]](/tpl/images/0401/5602/0be52.png)